Roche’s lampalizumab halts geographic atrophy
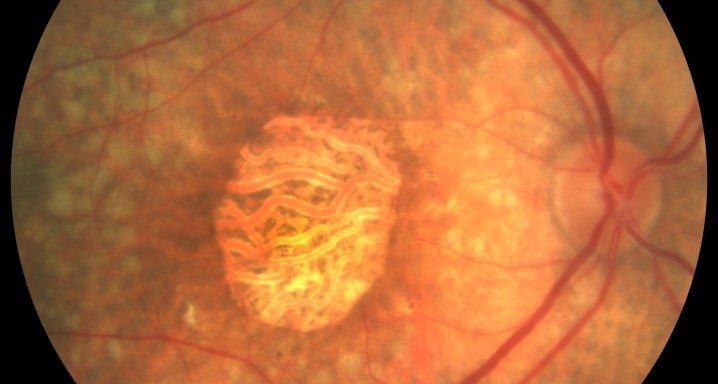
A publication in Science Translational Medicine shows that Roche has a rising star in the 15 million patient market of age-related macular degeneration (AMD). In a Phase II trial, US and German researchers showed efficacy in geographic atrophy, an advanced stage of AMD, which has currently no treatment.
One week prior to the publication, Roche announced it has initiated two Phase III trails (CHROMA and SPECTRI) enrolling 936 patients with the advanced form of AMD that affects 5 million AMD patients and has currently no cure. Primary endpoint is slowing for disease progression at 12 months, secondary endpoint is visual acuity at 24 months. However, rumors say the FDA could accelerate patient access through granting breakthrough status to the treatment.
In a multi-center, randomized, 18 month Phase study that recruited 129 AMD patients (MAHALO), lead author Brian Yaspan observed a 20% reduction in lesion area progression in patients receiving Roche/Genentech’s antibody drug candidate lampalizumab at acceptable safety profile. Lampalizumab zeroes in on complement D, part of the innate immune defense’s alternative complement pathway
Genome analysis of participants identified a patient subgroup with complement D variants who showed a 44% reduction in geographic atrophy area progression. The authors say targeting the alternative complement pathway has potential to be a viable treatment option for patients with secondary geographic atrophy.



 adobe.stock.com - ipopba
adobe.stock.com - ipopba BioDlink
BioDlink